
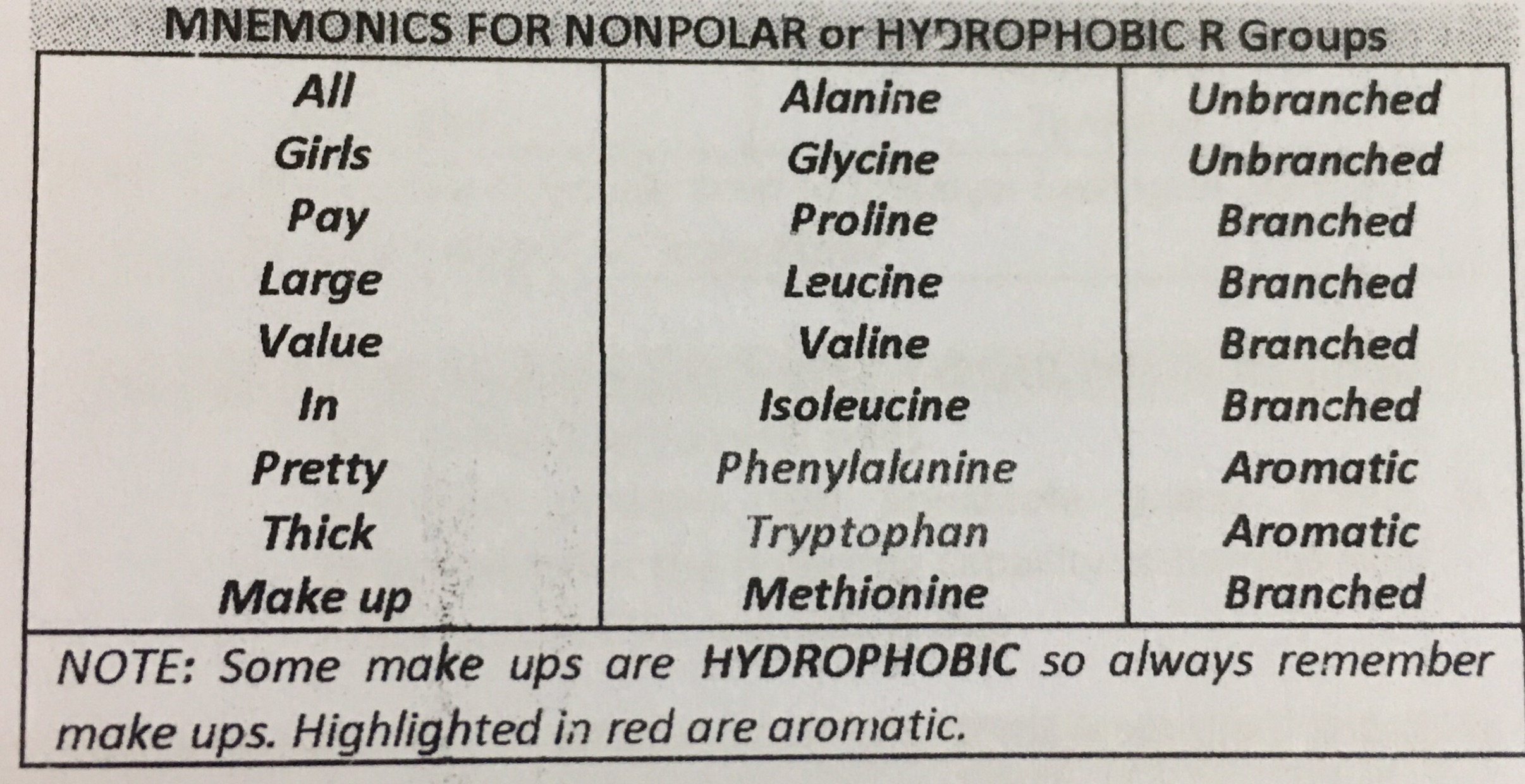
A single polypeptide chain may have different regions that take on different secondary structures. These include various loops, helices and irregular conformations.

While the a helix and b sheet are by far the most common types of structure, many others are possible. The major constituent of silk (silk fibroin) consists mainly of layers of b sheet stacked on top of each another. In this arrangement, side chains project alternately upward and downward from the sheet. Generally the primary structure folds back on itself in either a parallel or antiparallel arrangement, producing a parallel or antiparallel b sheet. Again, the polypeptide N-H and C=O groups form hydrogen bonds to stabilize the structure, but unlike the a helix, these bonds are formed between neighbouring polypeptide (b) strands. In a b sheet, the polypeptide chain folds back on itself so that polypeptide strands like side by side, and are held together by hydrogen bonds, forming a very rigid structure. The structure of a b sheet is very different from the structure of an a helix. An example of a protein with many a helical structures is the keratin that makes up human hair. The side chains project outward and contact any solvent, producing a structure something like a bottle brush or a round hair brush. The alpha helix has precise dimensions: 3.6 residues per turn, 0.54 nm per turn. All C=O and N-H groups are involved in hydrogen bonds, making a fairly rigid cylinder. Each carbonyl is linked by a hydrogen bond to the N-H of a residue located 4 residues further on in the sequence within the same chain.

In this conformation, the carbonyl and N-H groups are oriented parallel to the axis. An a helix, as the name implies, is a helical arrangement of a single polypeptide chain, like a coiled spring.


 0 kommentar(er)
0 kommentar(er)
